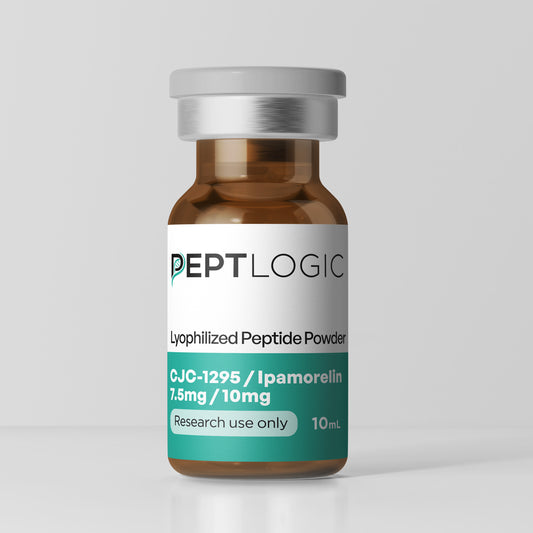
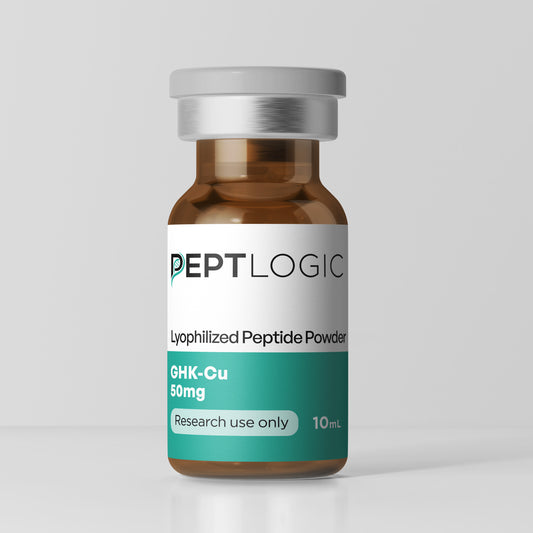
Clinical Grade Products You Can Trust
At PeptLogic, we provide extensive, third-party verification including costly endotoxin analysis to ensure LPS-free peptides and full sterility testing from our ISO 17025 accredited lab.
Endotoxins are invisible bacterial contaminants that can trigger unintended immune responses, even when no visible impurities exist. Our advanced testing protocol eliminates these risk of threats using medical-grade standards.
Every batch is backed by transparent certificates covering purity, content, endotoxin levels, and sterility. These safeguards aren’t extras—they’re essential.